COMPLIANCE
Quality assurance takes top priority for Jerusalem Pharmaceuticals. Our facilities are GMP certified by competent local and foreign authorities. Moreover, our facilities in Palestine and Jordan hold ISO 9001 and ISO 14001 accreditation.
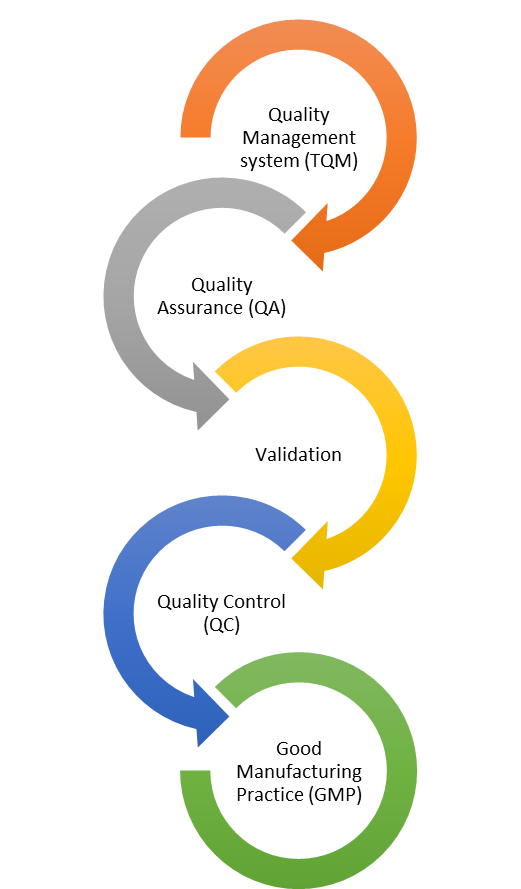
Through a well-established quality management system supported by an appropriate organizational structure, Jerusalem Pharmaceuticals pursues to build effective quality control and quality assurance throughout the production process in order to ensure efficacy, safety and convenience of its products. To this end, the responsibilities of the quality departments at its various facilities are key and have been distribute across three main units: quality control, quality assurance and validation.
Our quality departments boast a scientific tradition and several achievements, including:
- Published a number of scientific research papers in well-reputed journals including Photochemistry & Photobiology, International Journal of Pharmacy and Pharmaceutical Sciences, European Journal of Chemistry; International Journal of Pharmaceutical Compounding, Journal of Chromatographic Science, Pharmaceutical Chemistry Journal and Chromatographic Research International.In addition it is customary that our scientific team visits and participates in a number of local, regional and international conferences and exhibits.
- Developed numerous methods of analysis that were published in governed and well-esteemed pharmacopoeias and scientific journals. Few examples are included below.